 Since 2000, R&D spending has increased by more than 50 %
Since 2000, R&D spending has increased by more than 50 %
► The global spending appears since 2010 to have reached a plateau $68 Billion
► Time from discovery to market is still the same since decades : between 10 & 15 years
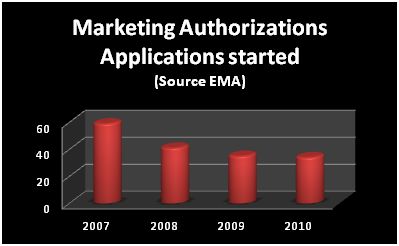
► New drugs output flat mirrors the R&D productivity crisis
► The current models for drug development are not efficient enough while there’s increasing demand for regulatory compliances and safety testing
►The number of candidates killed during costly late stage clinical trials has more than doubled from 26 in 2005-2007 to 55 in 2008-2010
 ► Among the 21 NMEs approved in 2010, only 30% come from major Pharmas (annual R&D budget > $2Billions)
► Among the 21 NMEs approved in 2010, only 30% come from major Pharmas (annual R&D budget > $2Billions)
Small/medium companies (including biotech) very often lack of specific knowledge and competencies on how to go to Phase I and it is therefore necessary to look for help at the very beginning. Preclinical advice as well as regulatory assistance is highly recommended, it clarifies the milestones in the development and the associated costs.
Before embarking on the way of nonclinical studies, it is worth to plan a meeting with the authorities to:
- Validate the pre-clinical development plan.
- Confirm the design of animal studies (animal model, selection of species).
- Confirm that the studies proposed are sufficient to support proposed clinical trials.
Meetings with regulatory authorities are often complex to manage, expensive and require in any case a careful preparation.
http://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2009/10/WC500004089.pdf
Whether or not you decide to face the meeting, the appropriateness of the development plans should be checked thoroughly for scientific and regulatory compliance to avoid wasting time and money.
